Opinions expressed by Forbes Contributors are their own. Blood pressure medication recalled over possibly containing cancer-causing impurity FDA made the assessment of Lupin Pharmaceuticals product after laboratory testing.
More than 30 batches of pills used to treat high blood pressure have been contaminated by an impurity that can increase the risk of cancer.
Blood pressure medication recalled due to cancer. Is being recalled by the US. NDEA and NDMA are industrial contaminants and both are on lists of chemicals suspected to cause cancer in people. Common blood pressure drug recalled due to cancer-causing chemicals.
Is voluntarily recalling its Irbesartan and Hydrochlorothiazide tablets at. The Food and Drug Administration said late last week that Lupin is voluntarily recalling certain dosages of irbesartan tablets and irbesartan and hydrochlorothiazide. At least five manufacturers are.
These medications are being recalled due to the presence of N-Nitrosodiethylamine NDEA or N-Nitrosodimethylamine NDMA. Blood pressure medication made by Lupin Pharmaceuticals Inc. Blood pressure drug Losartan recalled More blood pressure drugs are being recalled after being found to contain trace amounts of a potentially cancer-causing ingredient one of.
The FDA has published a new recall advisory from Lupin Pharmaceuticals over two different drugs. Cancer-causing chemicals spark recall of blood pressure drugs over contamination fears but patients have been warned not stop taking their medications. Tuesday 10th August 2021 951 am.
The recall has been issued by the Medicines and Healthcare products. Food and Drug Administration for possibly containing a cancer-causing impurityThe voluntary recall. ShakeOut Day to help prepare Tennesseans for earthquakes News Nashville-Davidson Tennessee.
More Blood Pressure Medication Recalls Due To Cancer Concerns. Blood pressure pills recalled over cancer risk. Is my medication being recalled.
Blood pressure and fluid retention drugs recalled over cancer concerns. Two types of blood pressure medication made by the company Lupin Pharmaceuticals are being recalled because they may contain high levels of a. The Medicines and Healthcare products.
Blood pressure medication recalled over risk of cancer-causing impurity Portland Oregon. Batches of blood pressure medication have been recalled due to potential risks of increasing likelihood of cancer. Medicines watchdog said that the recall is precautionary.
The FDA warned in late May and early June 2020 that some lots of extended-release metformin may contain unacceptable levels of N-nitrosodimethylamine NDMA a possible cancer-causing chemical. Fears that some drugs have been contaminated with a substance that could possibly increase the risk of cancer has seen certain blood pressure drugs recalled. First it was certain blood pressure and heartburn drugs.
Torrent Pharmaceuticals is expanding a recall of blood-pressure medication possibly tainted with a cancer-causing chemical. 2 blood pressure meds recalled for possibly containing a cancer-causing substance. Oct 20 2021 at 1059 am NPR.
Food and Drug Administration FDA. The Food and Drug Administration has recently recalled a number of blood pressure medications after discovering that they contained potential. Now some medications used to treat type 2 diabetes are being recalled because of cancer concerns.
Food and Drug Administration FDA. A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. Bill draws bright lines on student transcript access.
It is a precautionary measure to. Two types of blood pressure medication made by the company Lupin Pharmaceuticals are being recalled because they may contain high levels of a substance that could cause cancer. In another recall over cancer-causing impurity levels Lupin Pharmaceutical has recalled several batches of its Irbesartan tablets and Irbesartan and Hydrochlorothiazide tablets because N.
DOZENS of blood pressure pills have been recalled from pharmacy shelves because they contain a chemical linked to cancer. A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. FDA maintains the most current lists of drugs that are being recalled and those that are not included in the recall.

Hsa Recalls 3 Brands Of High Blood Pressure Drug Over Cancer Risk Today

Bph Blood Pressure Health Blood Pressure Maintenance Caplets Walgreens

Information Free Full Text Noninvasive Blood Pressure Classification Based On Photoplethysmography Using K Nearest Neighbors Algorithm A Feasibility Study Html
Pdf Clinical Practice Guideline For Screening And Management Of High Blood Pressure In Children And Adolescents
Blood Pressure Pills Recalled Over Cancer Risk The Independent

Pdf Determination Of Blood Pressure Percentiles In Normal Weight Children Some Methodological Issues

Recall Of Blood Pressure Medication Losartan Expands Over Cancer Concerns Everyday Health

How To Lower Blood Pressure Naturally Or With Medications

Hsa Recalls Three Brands Of High Blood Pressure Drug Losartan Over Cancer Risk Health News Top Stories The Straits Times

Understanding Blood Pressure Nps Medicinewise
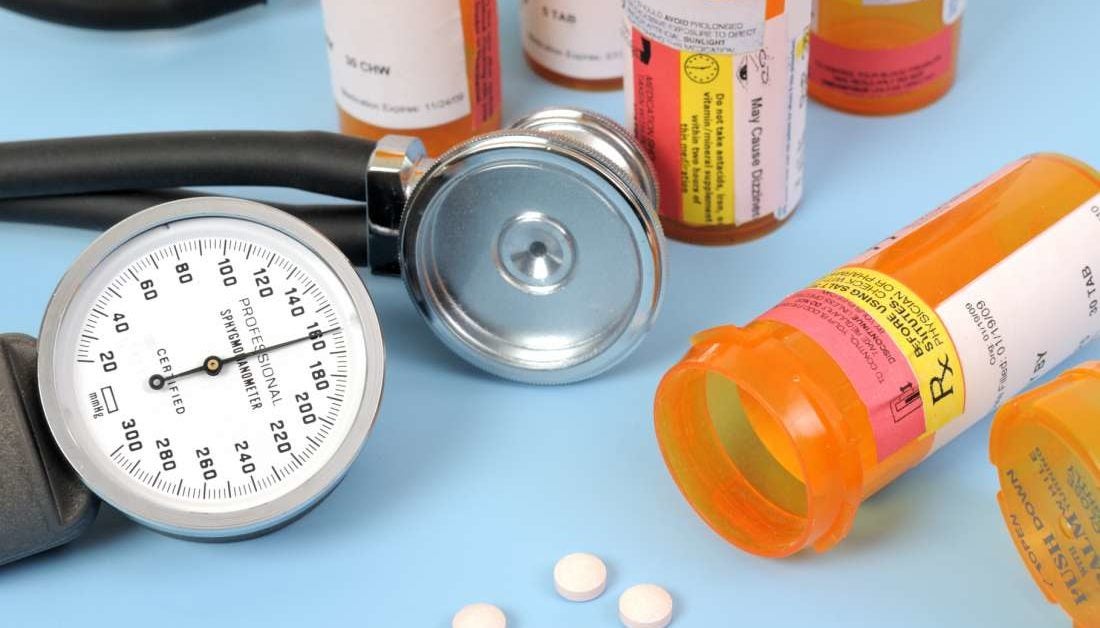
Death Risk Increased With Two Blood Pressure Drugs

Bar Graphs Showing Basal And Response Heart Rate And Blood Pressure Download Scientific Diagram
High Blood Pressure Drug Recall Expands Again Over Cancer Risk Slashgear

3 Brands Of High Blood Pressure Medication Losartan Recalled For High Levels Of Cancer Causing Contaminant Dr Siew Com
YOU MAY LIKE :