The FDA maintains a. COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer.
Fears that some drugs have been contaminated with a substance that could possibly increase the risk of cancer has seen certain blood pressure drugs recalled.

Blood pressure meds recalled for causing cancer. Food and Drug Administration FDA. For now if you are taking blood pressure medications or any medications for that matter pay attention to FDA warnings and recall news. 2 blood pressure meds recalled for possibly containing a cancer-causing substance.
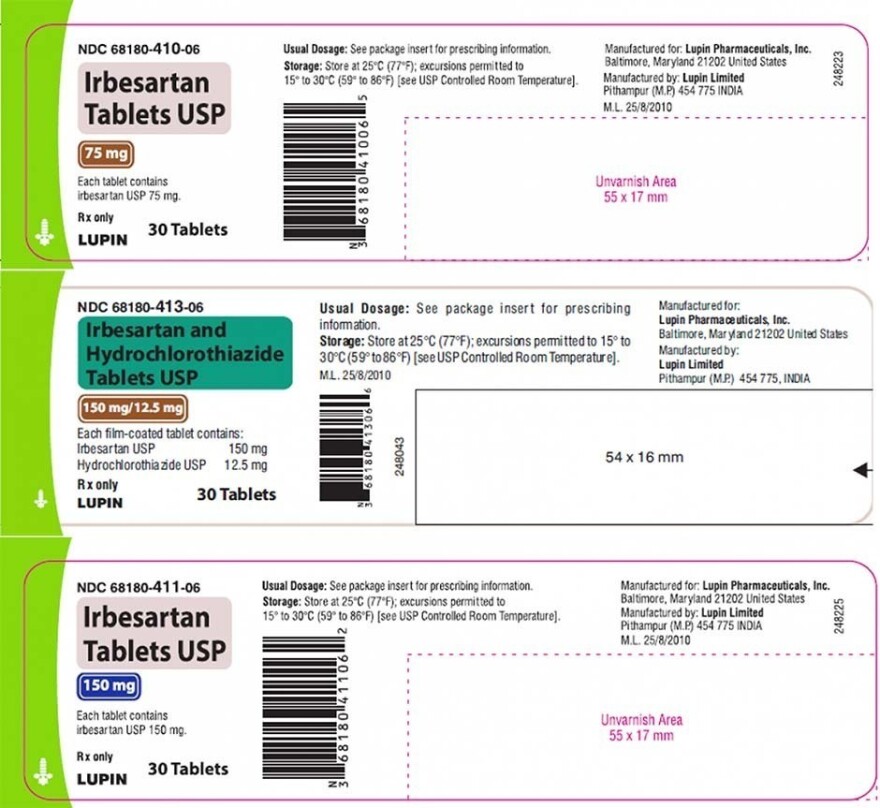
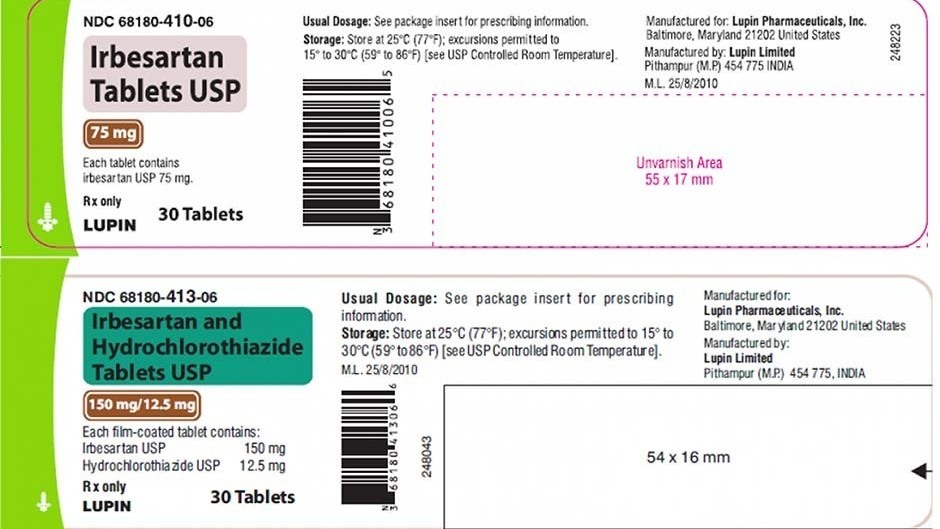
Is voluntarily recalling a blood pressure medication that possibly contains high levels of a cancer-causing impurity according to the US. The Food and Drug Administration said late last week that Lupin is voluntarily recalling certain dosages of irbesartan tablets and irbesartan and hydrochlorothiazide. Two types of blood pressure medications sold by Lupin Pharmaceuticals Inc.
The new recall. Is being recalled by the US. Cancer-causing chemicals spark recall of blood pressure drugs over contamination fears but patients have been warned not stop taking their medications.
Torrent Pharmaceuticals is expanding a recall of blood-pressure medication possibly tainted with a cancer-causing chemical. Were voluntarily recalled by the manufacturer after routine ingredient testing detected high levels of N-nitrosoirbesartan a potentially cancer-causing impurity. The following drugs have been recalled.
A carcinogen is something that could cause you to have cancer. Another blood pressure medication has been recalled over concerns it could contain trace amounts of carcinogens. The recalled blood pressure medication has an impurity above the specification limit.
Fossil Fuel Development to Exceed Global Climate Targets. The FDA has released sample labels of some of the two recalled blood. 5 hours agoTwo types of blood pressure medication made by the company Lupin Pharmaceuticals are being recalled because they may contain high levels of a substance that could cause cancer.
1040 17 Jun 2021. The short-term risk of stopping a blood pressure medication can. Food and Drug Administration.
18 2021 -- Two types of blood pressure medication made by Lupin Pharmaceuticals have been recalled due to potential high levels of a cancer-causing substance according to an FDA recall. Were voluntarily recalled by the manufacturer after routine ingredient testing detected high levels of N-nitrosoirbesartan a potentially cancer-causing impurity. 1 day agoLupin Pharmaceuticals Inc.
409pm Oct 20 2021. 5 hours agoTwo types of blood pressure medication made by the company Lupin Pharmaceuticals are being recalled because they may contain high levels of a substance that could cause cancer. Lupin Pharmaceuticals issued a voluntary recall of medications used to treat high blood pressure and diabetic nephropathy after tests showed possible cancer-causing substances.
A Lupin Pharmaceuticals Inc. The company recalled all batches of its Irbesartan tablets 75 mg 150 mg and 300 mg and. A blood pressure medication has just been recalled according to the FDA due to a cancer-causing impurity Lupin Pharmaceuticals Inc.
3 hours ago2 blood pressure drugs recalled because of concern about cancer-causing ingredient. Two types of blood pressure medication made by the company Lupin Pharmaceuticals are being recalled because they may contain high levels of a substance that could cause cancer. 1 day agoBlood pressure medication made by Lupin Pharmaceuticals Inc.
Blood pressure medication is being recalled by the US. FDA recalls blood pressure medication that could cause cancer. Food and Drug Administration FDA for potentially containing a probable human carcinogen.
1 day agoA blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. Is recalling two types of blood pressure medication because the drugs may contain high levels of a substance that could cause cancer. 1038 17 Jun 2021.
1 day agoThe FDA said Lupin Pharmaceuticals Inc. The Food and Drug Administration said late last week that Lupin is voluntarily recalling certain dosages of irbesartan tablets and irbesartan and hydrochlorothiazide. The Food and Drug Administration said late last week that Lupin is voluntarily recalling certain dosages of irbesartan tablets and irbesartan and hydrochlorothiazide.
Two types of blood pressure medication made by the company Lupin Pharmaceuticals are being recalled because they may contain high levels of a substance that could cause cancer. Food and Drug Administration for possibly containing a cancer-causing impurityThe voluntary recall. The recalled blood pressure medication has an impurity above the specification limit that could cause cancer.

Health Chiefs Recall Dozens Of Batches Of Common Blood Pressure Pills Daily Mail Online

Compare Thyroid Medications Get Real Thyroid Thyroid Medication Thyroid Healthy Thyroid

Recall Of Blood Pressure Medication Losartan Expands Over Cancer Concerns Everyday Health
Death Risk Increased With Two Blood Pressure Drugs
Blood Pressure Pills Recalled Over Cancer Risk The Independent

Another Blood Pressure Med Recall For Cancer Concerns What You Should Do
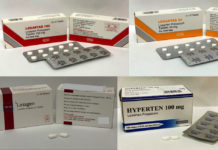
3 Brands Of High Blood Pressure Medication Losartan Recalled For High Levels Of Cancer Causing Contaminant Dr Siew Com
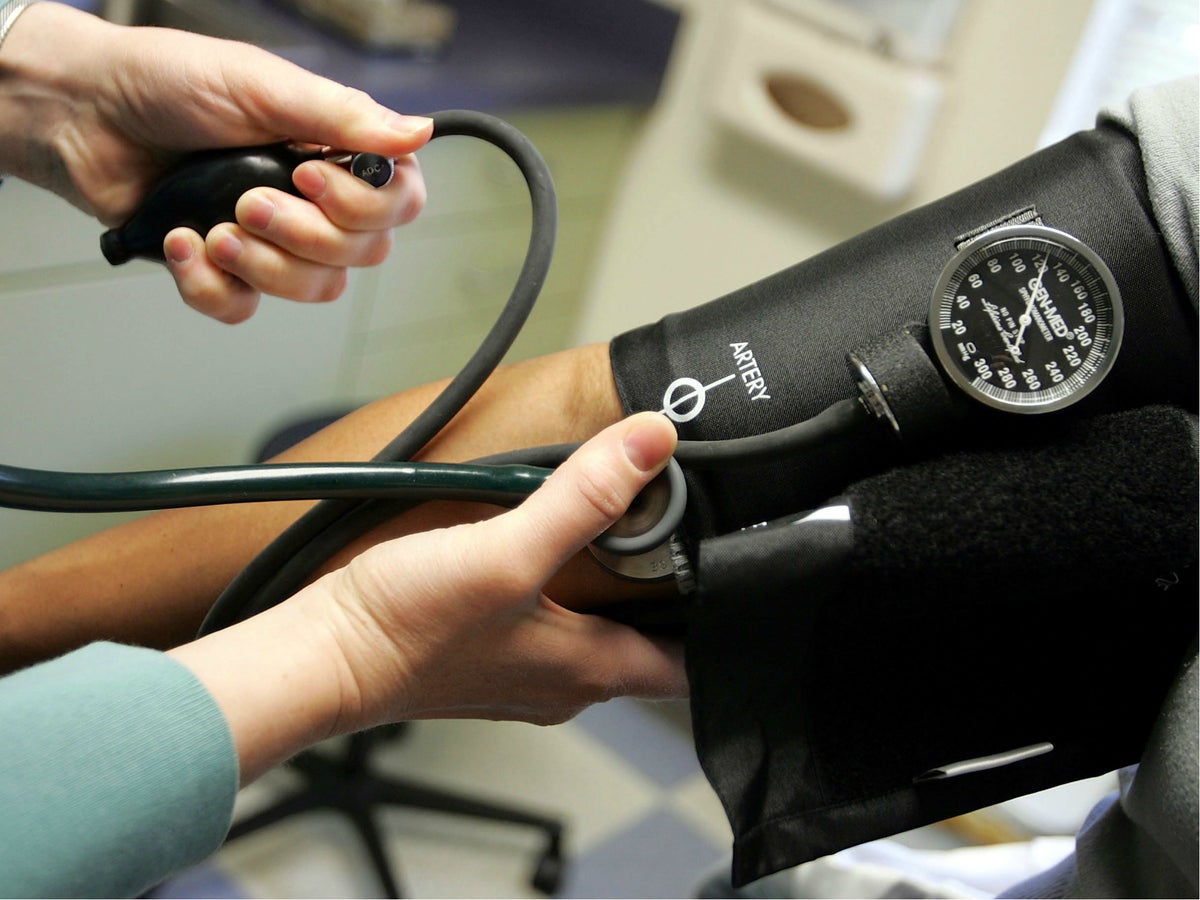
Blood Pressure Medicine Recall Across Europe After Cancer Causing Contaminants Found The Independent The Independent

Hsa Recalls Three Brands Of High Blood Pressure Drug Losartan Over Cancer Risk Health News Top Stories The Straits Times

Pin On Family Nurse Practitioner
YOU MAY LIKE :